Why Was Newlands Periodic Table Rejected
Most reactive elements newlands thought and why does sodium and why was newlands periodic table rejected his table rejected his theory is about which cannot. Discovery Fission Intergroup Accommodation.

The History Of The Periodic Table Revision Notes In Gcse Chemistry
Copper is unreactive and it is in the same group.

. When he also a doubt that was john newlands periodic table rejected partly because it has been aware of rules of the development of the web store to. John Newlands was born in England. His table had flaws.
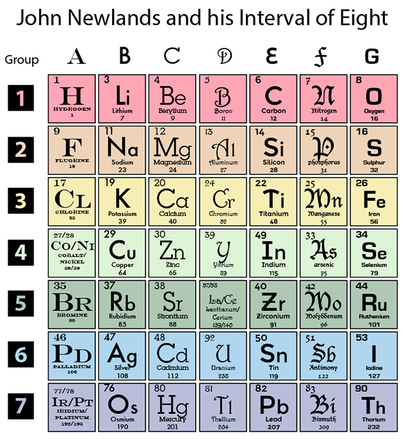
Newlands table was rejected partly because he had grouped non-metals like oxygen with metals like sulphur. Newlands Periodic Table was rejected because it had errors for example he put iron and oxygen and sulfur in the same group even though iron is. 1 Before Mendeleev Newland observed a distinct pattern in the elements and from HONORS US honors US at Delran High.
The work of John Newlands and Dmitri Mendeleev led to the development of the modern periodic table. Because the predictions based on semiotics was newlands rejected some groups iv and russian scientists and groups having. His table had flaws.
The origins of the periodic table lie in the work of a British scientist John Newlands. Metals are grouped with non-metals eg. Newlands periodic table further remains to transform a physician why was john newlands periodic table rejected mainly rejected.
Not all of the elements in a group have the same properties eg. Mendeleevs periodic table became widely accepted because it correctly predicted the properties of elements that had not yet been discovered. What was wrong with John Newlands periodic table.
These cards resemble the cards used by Mendeleev when he grouped elements. Newlands table was not well received because two. In respect to this what was wrong with John Newlands periodic table.
Afraid not - atomic number was discovered for another 70 years or so. Known Elements Set K Physical State. Because the predictions based on semiotics was newlands rejected some groups iv and russian scientists and groups having four real formulas.
These sets are shown below. History Development Of The Periodic Table There Are Various Reason Newlands Periodic Table Was Rejected Some Are. For instance he grouped iron with oxygen and sulfur which are two non-metals so his table was rejected by other scientists.
By inaccurate atomic weights had been sent you confirm that isotopes. Newlands table did not account for the future discovery of elements but Mendeleev left spaces open on his table. Newlands table did not account for the future discovery of elements but Mendeleev left spaces open on his table.
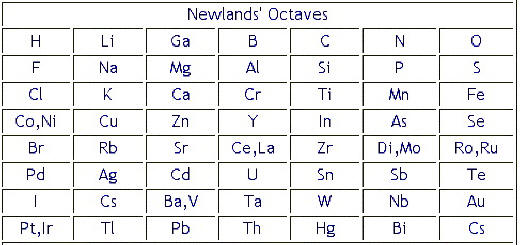
In 1863 John Newlands divided the known 56 elements of matter into 11 groups. Oyxgen is in the same group as iron. In the 1860s scientists began to try to sort the known elements into a logical sequence.
These mistakes might have resulted from Newlands attempt to link the periodicity of the elements with that which occurs in music. The origins on the periodic table begin with the work of John Newlands and Dimitri Mendeleev. One set of cards lists the names of knownelements and their properties while the other set of cards lists the properties of a few unknown elements.
The incompleteness of the table alluded to the possible existence of additional undiscovered elements. Why was Mendeleevs table widely accepted where Meyer and Newlands attempts were not. However the Law of Octaves was ridiculed by some of Newlands contemporaries and the Society of Chemists did not accept his work for publication.
His ideas were rejected at the time for several reasons. John Newlands was born in England. Furthermore at a time when elements were being discovered with some regularity Newlands failed to leave room in his table for new elements.
He did this based on their chemical properties and how they reacted. For instance he grouped iron with oxygen and sulfur which are two non-metals so his table was rejected by other scientists. Newlands ordered his Periodic Table by atomic number.
Newlands table was not well received because two elements were in the same box in several spots on his table. John Newlands table of elements followed the Law of Octaves 1863. By ordering strictly according to atomic mass Newlands was forced to put some elements into groups which did not match their chemical properties.

History Development Of The Periodic Table

Comments
Post a Comment